5 Reasons Why Cancer Patients Should Approach Clinical Trials with Caution

Clinical trials bring hope of a new treatment option but participating in them may not be the best way to overcome a roadblock in your cancer treatment. The new drugs or treatment options, tested in these trials at various cancer centers as part of their clinical research, are not equally effective for all the participants.

The focus of a clinical trial lies in determining the safety and efficacy of a new cancer treatment approach by observing the response of the participants in the research study. These clinical trials advance medical research in the field of oncology, but they are not designed to take into account the depth of every participant's specific set of cancer cell mutations which are critical for properly targeting treatment.

If your cancer has recurred despite standard treatments like chemotherapy, surgery, and radiation, then a clinical trial, even with the uncertainties involved, may seem like the only beacon of hope.
Fortunately, there are Centers of Excellence for cancer treatment like Envita Medical Centers who aim to remove these uncertainties and focus on precision-targeted cancer care based on patient-specific cancer genomic mutations. By using the N-of-1 model to establish treatment options, patients can receive the possible benefits of the latest medical innovations while avoiding the potential risks associated with large scale clinical trials.

The N-of-1 treatment model is based on the single subject with an individual patient as the sole unit of observation. Facilities like Envita Medical Centers approach care in this manner to identify patient-specific cancer mutations and design a medical blueprint to attack them from all angles. The goal is to find optimal treatment options for an individual patient, using objective data-driven criteria, so the patient can have the best chance to respond to care.
Envita follows the N-of-1 clinical trial model on all patients, irrespective of their cancer type, such as breast cancer, lung cancer, leukemia, prostate cancer, melanomas, or any other type of cancer. Patients with any type of advanced cancers or even those in their early stages, get access to N-of-1 clinical trial level of care at Envita.
Different Phases of Clinical Trials in the Classical Way
All clinical trials, in the classical way, are held in phases. The success of each phase determines whether the trial will go into the next phase or not. Once a trial completes all the three phases successfully, then the U.S. Food and Drug Administration (FDA) approves the treatment as a standard of care. A cancer clinical trial may take anywhere between 6 to 25 years.
Clinical Trial Phases
Reduced Phases of Clinical Trials in the Expedited Way
The classical approach to clinical development was Phase I, followed by Phase II, which then led to Phase III, but to tackle the issue of long-drawn phases of clinical trials some of the recently approved cancer drugs and therapies have gone through FDA established expedited programs. In fact, there's a steep rise in anticancer drugs and biologics approval via the expedited programs, over the years. The expedited programs called Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review, were introduced to address the need for new drugs and therapies required for treating life-threatening conditions like cancer. The programs cater to unmet medical needs, increased survival rates, decreased morbidities, and cut the FDA review period of a drug.
In this expedited way, development plans are more fluid essentially deferring the phase III of the classical approach, so trial time may take anywhere between 2 to 5 years from conception to approval. Bypassing phase III shifts a substantial amount of drug evaluation into routine clinical practice where there is less attention to collecting patient outcome data. Patients involved in these expedited clinical trials are subjected to phases where the safety and efficacy of the new therapy is being established (essentially phases I and II of the classical approach).
Given the different phases and aspects of a clinical trial, it may not always be your best bet. Cancer is still the second-leading cause of death in the United States, so choosing the right option may make all the difference between life and death.
1. You may not receive the new treatment in the classical approach or experience potential adverse effects in the expedited approval approach
If you enroll for a clinical trial of a new treatment in phase III, where many participants are involved, then you may end up in the control group. In the control group, cancer patients are given the existing treatment, if not a placebo, so their responses can be compared to those in the experimental group receiving the new treatment.
According to the National Cancer Institute (NCI), which is a part of the National Institutes of Health (NIH), a placebo is designed to look like the medicine being tested, but it is not active. Placebos are rarely used in cancer treatment clinical trials. They may be used when there is no standard treatment or in a clinical trial that compares standard treatment plus a placebo, with standard treatment plus a new treatment.

There is no way to know whether you will be in the control group or the experimental group because most studies are randomized controlled trials (RCT). Participants are randomly assigned to the control or experimental group, and you don't have a say in it. In some cases, the trials are double blinded so neither the researchers nor the participants know who's getting which treatment, till the researchers analyze participants' responses.
If you are participating in an expedited clinical trial program, then you are getting the new therapy but there are risks of a potential side-effect. A 2016 study shows many drugs that went through the expedited clinical trial approach produced adverse effects like severe skin reactions, and some of these drugs even had to be withdrawn from the market due to these adverse events.

How to overcome this challenge:
If you seek treatment at a facility like Envita Medical Centers, where they follow the N-of-1 approach, you are essentially in a single-patient clinical trial. You are not getting the standardized treatment available at other hospitals based on your cancer stage and type. Instead, you are receiving an exclusive treatment regimen designed specifically for you considering your specific genomic mutations. In this model, the doctors closely monitor your responses to care and modify treatment as needed without being hindered by clinical trial-based restrictions.
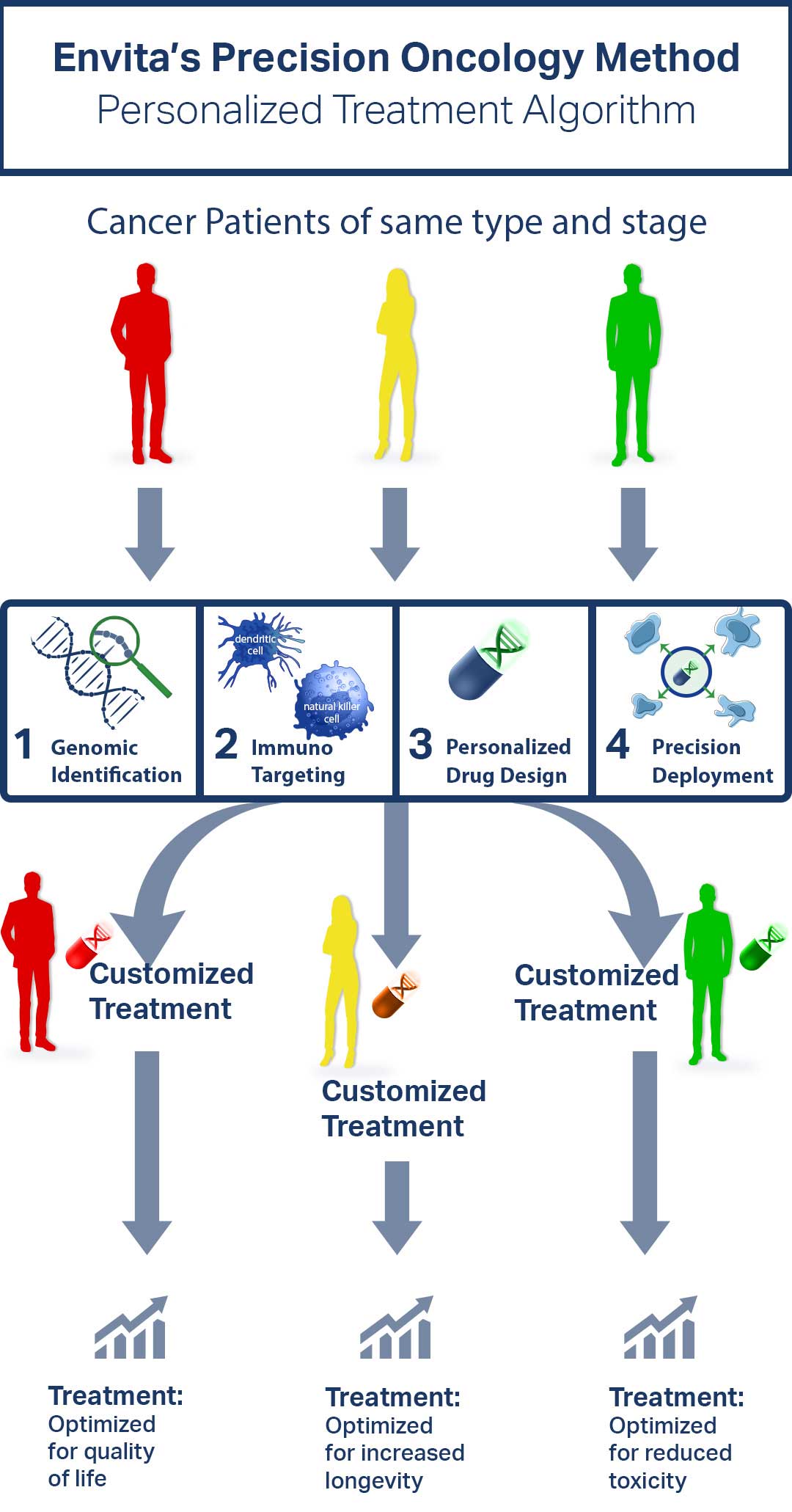
Under Envita Medical Centers' care you are receiving targeted therapies based on sophisticated algorithms, which have been developed over 25 years of clinical experience treating complex and difficult cancer cases.
Envita's proprietary testing allows their expert team including certified oncologists, interventional radiologists, researchers, and pharmacists to design a protocol based on your individualized needs. They combine the most advanced technologies in conventional care with research-based adjuvant therapies, using an integrative approach to help you gain an edge over your cancer.
2. Uncertainty involved
Since clinical trials are a part of the process to determine whether certain interventions are effective or not, the uncertainty always remains. Even with the same type and stage of cancer, different individuals may have different responses to the same treatment. Since cancer is a disease of genetic mutations, every individual's response to treatment varies. For some, clinical trials may be very effective, while for others it may not be as effective. The success of a clinical trial is determined by observing the responses of the majority of its participants, so the focus is not on any individual cancer patient but instead what works for the masses.
The risks involved are even more if you are in phase I or II, because the drug or intervention procedure is in its initial phase of testing. Though in most cases oncologists refer patients to phase III of clinical trials, when the efficacy of the treatment has been established, still the risk of failure looms large. According to a study published in the American Association for Cancer Research (AACR), phase III randomized controlled trials (RCT) in oncology fail in leading to registration of new therapies more often than RCTs in other medical disciplines. In such cases, clinical trials may do more harm than good.
How to overcome uncertainty:
Your chance of a favorable outcome increases when your treatment regimen is focused on you. Envita goes way beyond standard care to deep dive into your cancer and identify treatment targets to customize a protocol targeting your specific cancer cell biomarkers. Biomarkers are attributes of cancer cells containing specific targets.
Envita's precision algorithm considers each patient's individual genomics, proteomics, metabolomics, and immuno targets. Analyzing all these factors helps the healthcare providers at Envita Medical Centers gain a better understanding of the root cause of your cancer, so they can personalize a treatment plan to target your specific cancer drivers. This treatment approach not only attacks the existing cancerous cells, but also aims to prevent its metastatic spread.
Envita Precision Algorithm vs.
Standard Oncology Precision Testing
-
Envita Medical Centers
-
Standard Oncology
Precision Testing - RNA Transcriptome Genes
- Envita Medical Centers: 20,000+
-
Standard Oncology:
Unchecked
- SNV/CNV Genes
- Envita Medical Centers: 452
- Standard Oncology: 309
- Rearrangements/Fusion Genes
- Envita Medical Centers: 51
- Standard Oncology: 27
- Microsatellite Instability (MSI)
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Checked
- Tumor Mutation Burden (TMB)
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Checked
- BRCA 1/2
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Checked
- Immunohistochemistry
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Chemosensitivity
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Concurrent Liquid Biopsy
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Exosomal miRNA Analysis
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Circulating Tumor Cells Enumeration
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Pharmacogenomics
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Individualized Therapy Recommendation
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- CarcinogenicExposure - Root Causes
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Inflammation Markers
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
- Metabolic Target Drivers
-
Envita Medical Centers:
Checked
-
Standard Oncology:
Unchecked
*Individual results may vary. Envita makes no guarantees for outcomes.
While standard genetic testing analyzes a limited number of markers for which commonly available drugs are referenced for treatment, Envita's advanced genomic analysis includes over 20,000 biomarkers and molecular profiles, based on many parameters like the RNA transcriptomes, to establish targets for treatment. In addition, the precision targets identified require custom-built medications specific to the patient to help turn the tide in their treatment and put them on a faster route to recovery.
3. Lack of a multi-dimensional treatment approach
Clinical trials are undertaken to measure the efficacy of a certain treatment, so participants will receive a new drug or an intervention. The new drug or intervention may or may not be effective for all the participants in the group. If it's not effective, then you may need a multi-dimensional approach to attack your cancer cells from all possible angles through different therapies and interventions.
How to overcome this challenge:
Your cancer needs aggressive targeting from all possible angles, so it doesn't get a chance to grow or spread. Envita Medical Centers uses a multi-dimensional approach to precision-target your cancer cells, while strengthening your immune system to heal you holistically. They use cutting edge immunotherapies like MiRNA gene silencing to fix disparities at a molecular level, preventing the growth and spread of cancer cells.

Advanced treatments like GTFC™ (Genetically Targeted Fractionated Chemotherapy) increases the medicine-absorption capacity of the cancer cells by utilizing genetic testing to discover which agents will most likely respond to your cancer. This information allows smaller doses of targeted chemotherapeutic agents, along with cancer adjuvant agents, to attack your unique cancer cell biomarkers, increasing your chances of a better outcome. Lower doses of targeted medicine reduce the impact on healthy cells, so your immune system does not get weakened the way it does with maximum therapeutic dose chemotherapy. A strong immune system gives you an upper hand in your fight against cancer.
Additionally, Envita has also developed a non-invasive surgical option called CIPT™ (Chemo Immuno Precision Injections). This interventional radiology procedure is used to ablate large cancerous masses and strengthen your immune system with precision drug deployment.

As part of this procedure, genetically targeted chemotherapy, immunotherapy, and chemo adjunctive agents are placed inside a micro-catheter, thinner than a strand of hair. This catheter is then pushed through the arterial blood supply to directly reach inside the tumor and destroy it from the inside out.
At Envita, the opportunities are endless. Their expert team uses a combination of different treatment options to boost the chances of better outcomes. Here the focus is on the patient, not on the need to prove the efficacy of a certain treatment option.
4. Eligibility criteria may be a constraint
You must fail at least a couple of cancer treatments, even to be considered for a clinical trial. Then you must fit into the specific eligibility criteria set by the research team of each clinical trial. They have certain specifications like age, the type and stage of cancer, past treatment, and other medical conditions. Based on all these factors, you may or may not be eligible for a particular clinical trial.

Waiting for the right clinical trial, ideally suited for you may affect survivorship when you are dealing with a life-threatening condition. You can't afford to let the disease progress because your life is at stake here.
How to overcome this constraint:
You may ask your oncologist to inform you about any upcoming clinical trial best suited for you. Alternatively, you could seek a second opinion and treatment from the expert team at Envita Medical Centers. Since Envita follows the N-of-1 clinical trial model in their treatment regimen, you are getting access to a precision, comprehensive regimen designed specifically for you. You don't have to wait to meet certain criteria to receive the most advanced and holistic cancer care possible.
5. Limited focus
There are various facets to cancer treatment, like diagnosis, treating the cancer, long-term care, development of vaccines, and cancer prevention. Different clinical trials are conducted to focus on each of these different facets, so each trial's focus is limited. One clinical trial can only focus on any one of the different facets of care. Here are the different purposes of clinical trials in cancer:
- Trials for experimental treatments, new combinations of medicines, surgery, or radiation therapy.
- Trials to prevent the occurrence or recurrence of the disease.
- Trials to discover new diagnostic processes and screening mechanisms.
- Trials to improve quality of life when diagnosed with cancer.
Since you cannot participate in more than one clinical trial at a time, you may be missing out on comprehensive care.
How to overcome the limited focus:
Most people want to participate in a clinical trial with the goal of going into long-term remission, so they can live without the fear of cancer recurrence. The expert team at Envita Medical Centers also has the same goal, which is why they follow the N-of-1 clinical trial model in their treatment plan. Their researchers are constantly working on advanced medical technologies to enhance the efficacy of cancer treatment. They use the best in conventional medicine along with advanced phytotherapeutic drugs to improve quality of life, patient longevity, and reduce toxicity. Many of Envita's cancer patients feel so rejuvenated even before the treatment is completed, that they are able to go out hiking and indulge in other outdoor activities, which would typically not be an option when pursing traditional cancer care or even clinical trials.
The medical team uses a combination of various cutting-edge, proprietary treatment methodologies to potentially boost cancer kill and prevent its recurrence. Their diagnostics and screening mechanisms deep-dive way beyond conventional standards. They get a detailed understanding of each patient's unique cancer cell mutations, genetic targets, and messengers feeding the cancer. Armed with this information they are better equipped to aid you in combating your cancer holistically.
Take a tour of Envita Medical Centers
Envita Center of Excellence for Precision Oncology has over 25 years of experience treating complex and refractory to treatment cancer diagnoses. Most patients come to Envita after standardized oncology options have failed to yield lasting results. Since we follow the N-of-1 treatment model, we treat each patient as a single unit clinical trial offering them the best of our diagnostics and technologies to improve outcomes. For any of your cancer related queries, please feel free to call us at 866-830-4576.